Clinical Evaluation
Design & Engineering
Mechanical Design
Rapid Prototyping
Reverse Engineering
FEM Simulation
Quality and Validation
Quality System Support
VMP - DQ IQ OQ PQ Validation
Standard Operative Procedures (SOP) (IOP)
Technical File / Design Dossier
Design Control
DHF Design History File
Risk Management Process
Clinical Evaluation
Testing
Mechanical Testing
Analysis on Plastics
Biological Safety Evaluation
With our team of scientists, engineers and statisticians with many years of national and international experience in clinical, preclinical and technical areas we can support Your company in performing Clinical Evaluation, the assessment and analysis of clinical data pertaining to a medical device in order to verify the clinical safety and performance of the device.
Clinical evaluation is an ongoing process conducted throughout the life cycle of a medical device.
When placing a medical device on the market the manufacturer must have demonstrated through the use of appropriate conformity assessment procedures that the device complies with the relevant Essential Requirements covering safety and performance. Generally, from a clinical perspective, it is expected that the manufacturer has demonstrated the device achieves its intended performance during normal conditions of use and that the known and foreseeable risks, and any adverse events, are minimised and acceptable when weighed against the benefits of the intended performance, and that any claims made about the device’s performance and safety (e.g. product labelling and instructions for use) are supported by suitable evidence.
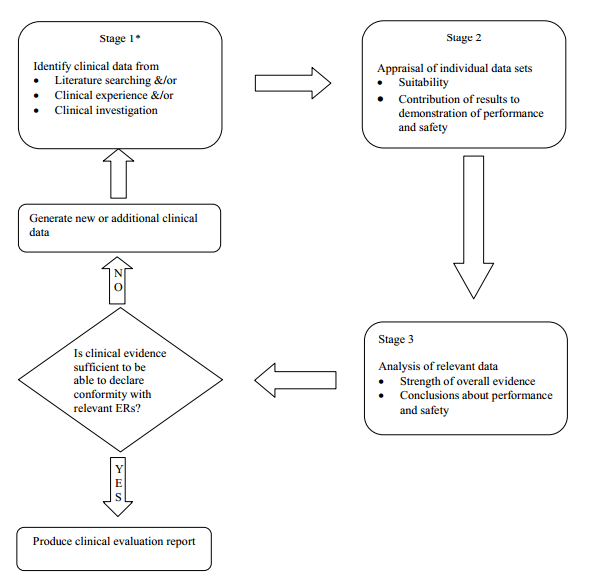
Once the scope has been defined, there are three distinct stages in performing a clinical evaluation:
• identification of pertinent standards and clinical data;
• appraisal of each individual data set, in terms of its relevance, applicability, quality and clinical significance; and
• analysis of the individual data sets, whereby conclusions are reached about the performance, safety and presentational aspects (labelling, patient information and instructions for use) of the device.
Sources of data/documentation used in a clinical evaluation can be collected from:
- Data generated through literature search
- Data generated through clinical experience
- Data from clinical investigations
Data generated through literature search
Literature searching can be used to identify published clinical data that is not in the possession of the manufacturer that may assist the manufacturer to establish acceptable performance and safety of a medical device. The data generated through literature searching may relate directly to the device in question (e.g. reports of clinical investigations of the device in question that have been performed by third parties, adverse event reports) or to equivalent devices.
Data generated through clinical experience
These types of clinical data are generated through clinical use that is outside the conduct of clinical investigations and may relate to either the device in question or equivalent devices.
Such types of data may include:
• manufacturer-generated post market surveillance reports, registries or cohort studies (which may contain unpublished long term safety and performance data);
• adverse events databases (held by either the manufacturer or Regulatory Authorities);
• data for the device in question generated from individual patients under compassionate usage programs prior to marketing of the device;
• details of clinically relevant field corrective actions (e.g. recalls, notifications, hazard alerts).
Data from clinical investigations
Clinical investigations are generally expected to be designed, conducted and reported in accordance with EN ISO 14155, Parts 1 and 2, Clinical Investigations of Medical Devices for Human Subjects, or to a comparable standard, and in compliance with local regulations.
It is recognised that where manufacturers source clinical investigation data reported in the scientific literature (i.e. investigations of either the device in question or equivalent devices that are undertaken by a third party), the documentation readily available to the manufacture for inclusion in the clinical evaluation is likely to be no more than the published paper itself.
The product literature and instructions for use should be reviewed to ensure they are consistent with the data and that all the hazards and other clinically relevant information have been identified appropriately.
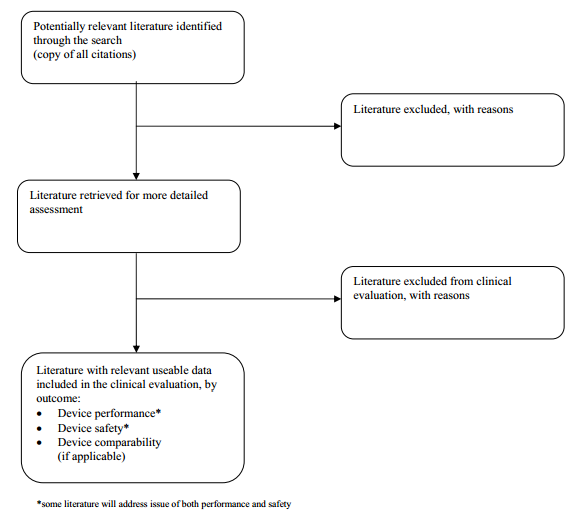
A POSSIBLE METHODOLOGY FOR DOCUMENTING THE SCREENING AND SELECTION OF
LITERATURE WITHIN A LITERATURE SEARCH REPORT