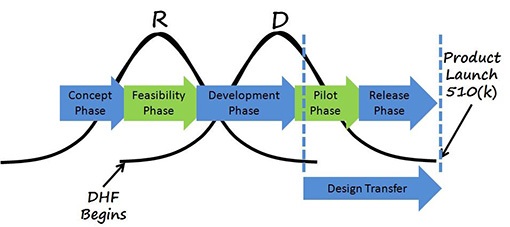
DHF Design History File
Design & Engineering
Mechanical Design
Rapid Prototyping
Reverse Engineering
FEM Simulation
Quality and Validation
Quality System Support
VMP - DQ IQ OQ PQ Validation
Standard Operative Procedures (SOP) (IOP)
Technical File / Design Dossier
Design Control
DHF Design History File
Risk Management Process
Clinical Evaluation
Testing
Mechanical Testing
Analysis on Plastics
Biological Safety Evaluation
The DHF contains or references the records necessary to demonstrate that the design was developed in accordance with the approved design plan and the requirements of the FDA's design control.
The United States Code of Federal Regulations Title 21 defines it as follow:
- § 820.30(j) Design history file."Each manufacturer shall establish and maintain a DHF for each type of device. The DHF shall contain or reference the records necessary to demonstrate that the design was developed in accordance with the approved design plan and the requirements of this part.
- § 820.3(e) "Design history file (DHF) means a compilation of records which describes the design history of a finished device."
Formal Design Control and Design History File (DHF) requirements (both a part of GMPs) were instituted by the FDA in 1996-1997. Not only are these requirements "must dos" because of regulations but they serve companies in the retention of their IP (intellectual property) via the proper documentation of development and changes and via the creation of false starts records, blind alleys and similar development "dead ends." If done properly, it can provide a valuable training tool for engineering, manufacturing, QA and marketing personnel.
The completed product DHF shall include the following:
- Detailed design and development plan specifying design tasks and deliverables as a "living document," usually in several iterations;
- Copies of approved design input documents and design output documents;
- Documentation of design reviews;
- Verification and validation documentation; and
- Copies of controlled design documents and change control rationale and records, when applicable.
Development documentation also needs to be properly witnessed where appropriate and thus may also serve in supporting patent claims.
Design and development contracts should explicitly specify the manufacturer's right to design information, as well as define and establish standards for the form and content of design documentation. This would include custom software as well.
Finally, certain basic design information may be maintained in a single project file in a specified location or a basic DHF document with pointers to other supporting documents not directly/physically included in the DHF (including dates / revision numbers).